half life formula for first order reaction
And so heres our equation for the half-life for a second order reaction. The half-life of a chemical reaction denoted by t 12 is the time taken for the initial concentration of the reactants to reach half of its original value.
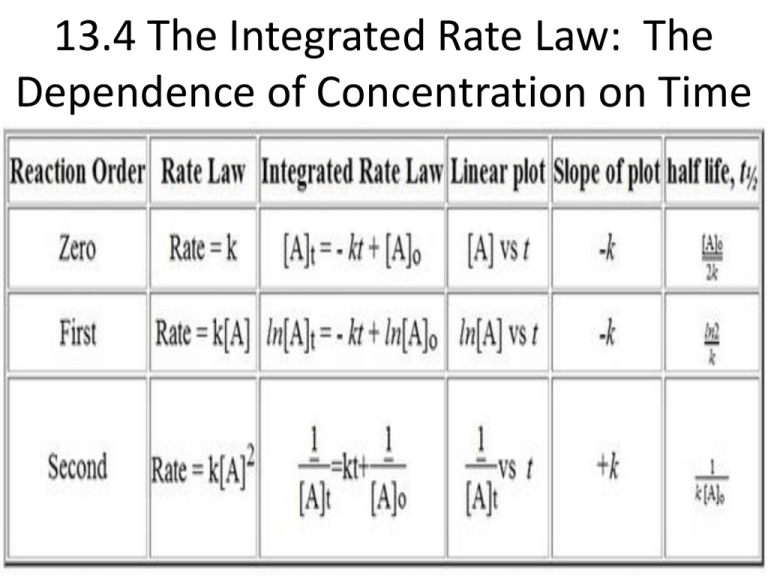
13 4 The Integrated Rate Law The Dependence Of Concentration On
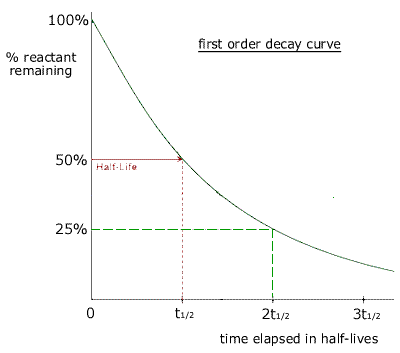
Half-life or t½ is the time that elapses before the concentration of a reactant is reduced to half its initial value.

. K 1 2303t log a a x. The half-life of a first-order reaction is a constant that is related to the rate constant for the reaction. The half-life of a first-order reaction does not depend upon the concentration of the reactant.
By rearranging the above equation the half-life of a first-order reaction can be obtained to be t_12frac2303klogleft 2 right t_12frac0693k. The half-life of a second-order reaction is given by the formula 1kR 0. Converting a half life to a rate constant.
It is a constant and related to the rate constant for the reaction. The half-life of a first-order reaction is given as t 12 0693k. The half-life of a second-order reaction is given by the formula 1kR 0.
Determining a half life. For a zero order reaction A products rate k. For first order reaction.
Therefore At t t 12 A A 0 2. What is the half-life of a first-order reaction with a rate constant of 25010-4 s-1Express your. Half-Life of a First-Order Reaction.
The half-life equation for a second-order reaction is t121kA0 t 1 2 1 k A 0. Graphical relations and half lives. T ½ 1 k A o Top.
I Where k 1 First order rate constant. The half-life equation for a zero-order reaction is t12A02k t 1 2 A 0 2 k. The half-life of a first-order reaction is given as t 12 0693k.
FraclnA_0A_tkt tfraclnA_0A_ttimesfrac1k. Where The half-life of a reaction is referred to as t 12 unit - seconds The initial reactant concentration is referred to as R 0 in molL. Calculate the half-life of the first-order reaction C 2 H 4 O g C H 4 g C O g if the initial pressure of C 2 H 4 O g is 80 mm and the total pressure at the end of 20 minutes is 120 mm.
For a first-order reaction the half-life is independent of concentration and constant over time. What is the half-life equation for a second-order reaction. So we get the half-life is equal to one over k times the initial concentration of A.
What is the expression for Half-Life of a First Order ReactionHere I derive it from the integrated rate lawThe answer is t ln 2 kAsk me questions. Since the reaction order is second the formula for t12 k-1A o 1. For a first order reaction t½ 0693 k and for a second order reaction t½ 1 k Ao.
Ln 12A0A0 -kt12. Notice this is very different for the half-life for a first order reaction. What is the half-life formula for a first order reaction.
Find Age of Substance From Given Half Life Exponential Decay. Where t12 is the half-life in seconds s and k is the rate constant in inverse seconds s-1. T ½ 0693 k For a second order reaction 2A products or A B products when A B rate kA 2.
Just divide both sides by k. Ln 2 0693 kt12. T 12 0693k.
The rate equation for first order reaction is-. For the first order reaction you can plug the definition of the half life into the concentration-time reaction to obtain a neat relationship. Hence the half-life period for zero order reaction depends upon the initial concentration of reactants.
For a first order reaction we saw that the half-life was constant but here the. T ½ A o 2k For a first order reaction A products rate kA. Notice that the half life does not depend on the reactant concentration.
This means that the half life of the reaction is 00259 seconds. Using half life to determine the age of a piece of coal. The half-life of a reaction is the time required for the reactant concentration to decrease to one-half its initial value.
Substituting the values in the expression for the rate constant of half-life first-order reaction the. Equations for Half Lives. The first-order reaction half-life equation is given by k 2303 t l o g R 0 R From the definition of the half-life of a first-order reaction at t t12 and R R 02.
Half Life of First Order Reactions First-Order Reactions We can derive an equation for determining the half-life of a first-order reaction from the alternate form of the integrated rate law as follows. Half-life equation for first-order reactions.

First Order Elimination Rate Constant And Half Life A Closer Look Lect 11 Youtube
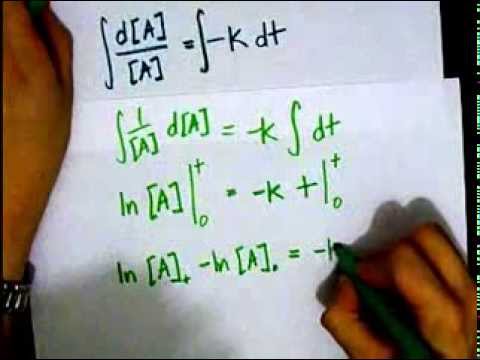
Integrated Rate Law First Order Reaction Youtube
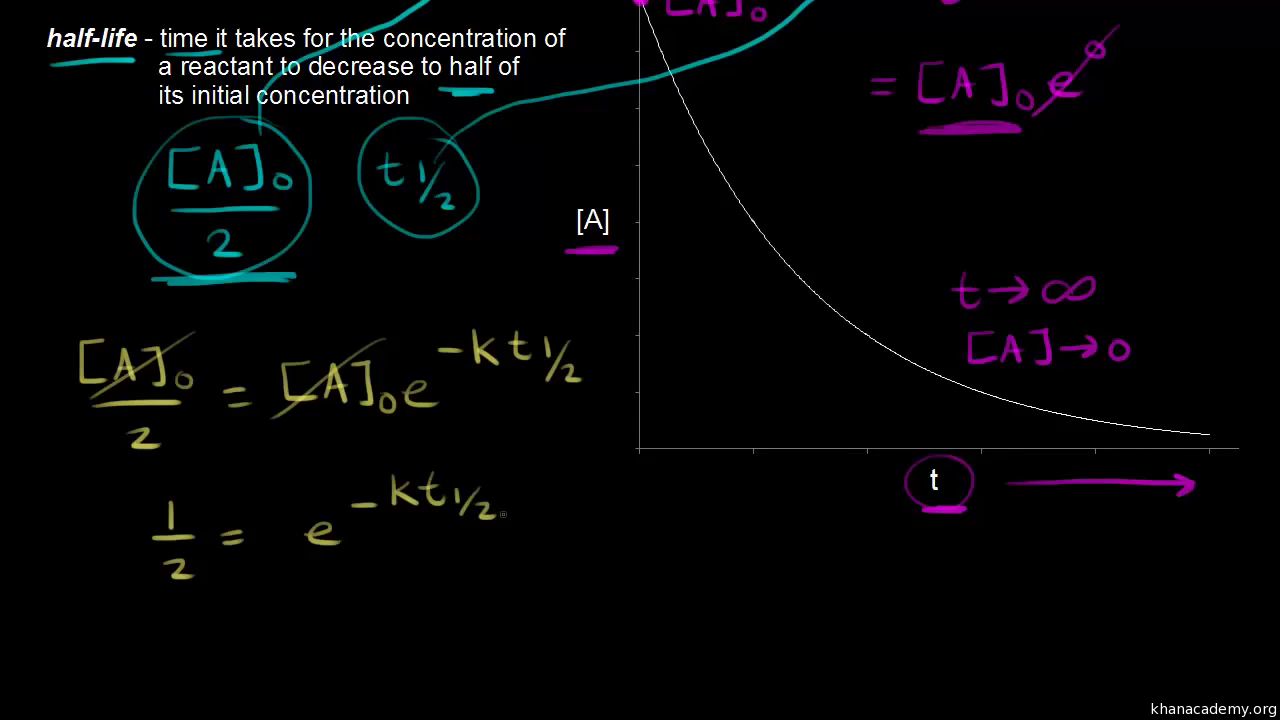
Half Life Of A First Order Reaction Video Khan Academy
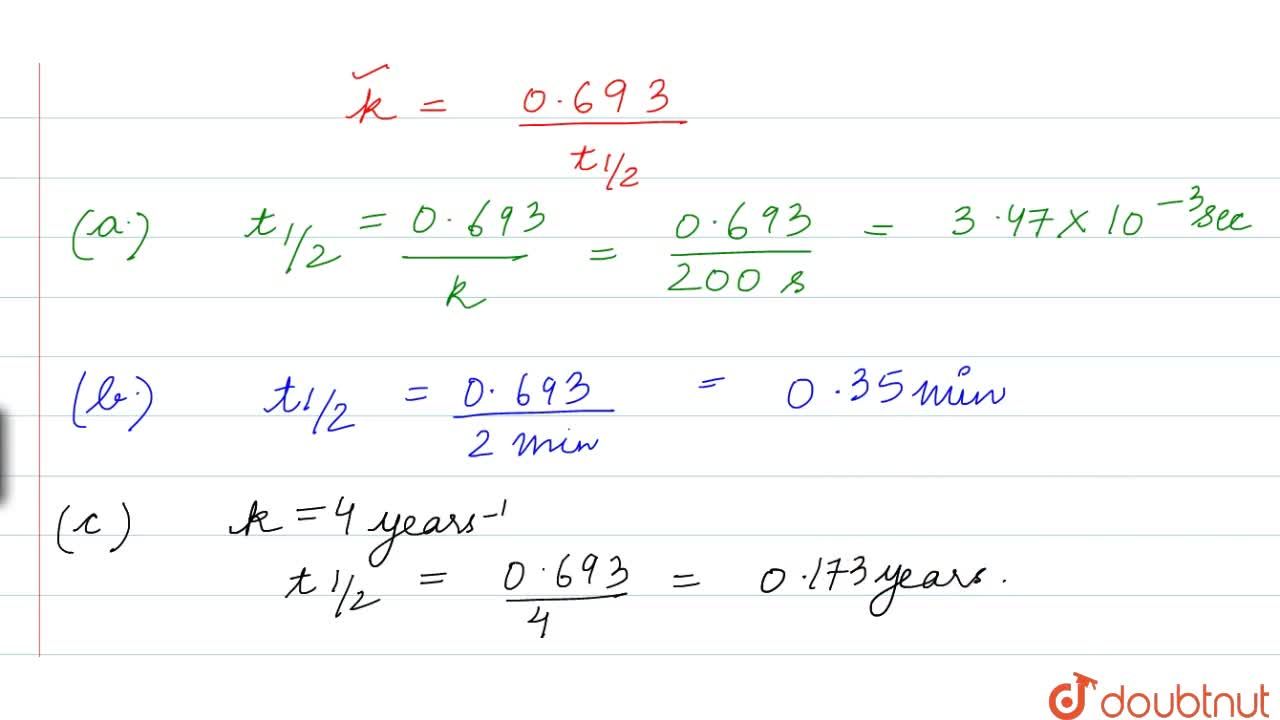
Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200s 1 B 2 Mi N 1 C 4years 1

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200 S 1 B 2 Min 1 C 4 Year 1

Integrated Rate Laws Zero First Second Order Reactions Chemical Kinetics Youtube

Half Life Of A First Order Reaction Video Khan Academy

First Order Reaction Definition Example Half Life Period Chemist Notes
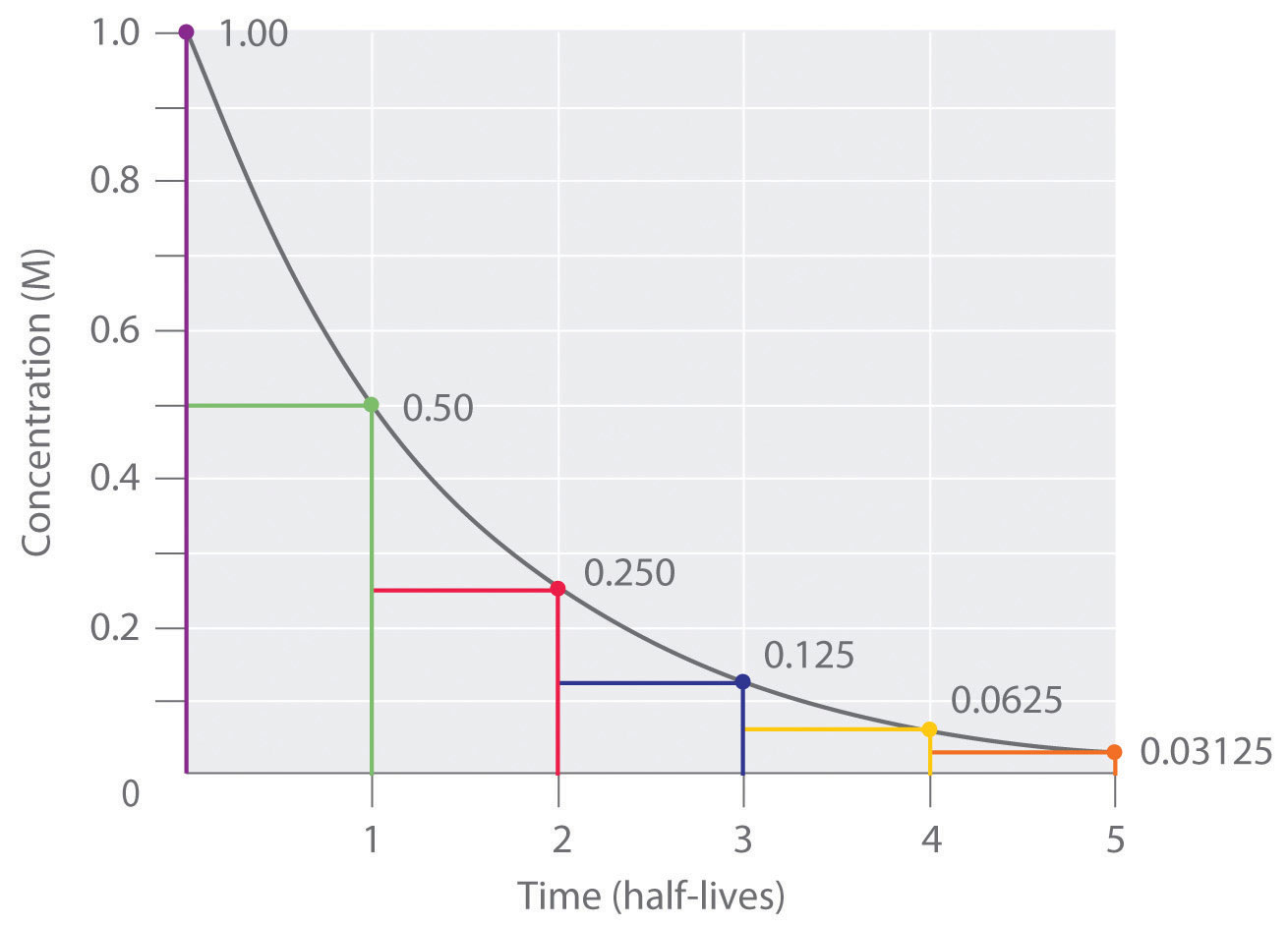
4 5 First Order Reaction Half Life Chemistry Libretexts

Using The First Order Integrated Rate Law And Half Life Equations Worked Example Video Khan Academy

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200 S 1 B 2 Min 1 C 4 Year 1
Zero Order Reactions Video Kinetics Khan Academy

First Order Reaction Definition Examples And Equations

Organic Chemistry Half Life And Shelf Life Of Second Order Reaction Chemistry Stack Exchange
Half Life Chemistry Problems Nuclear Radioactive Decay Calculations Practice Examples Youtube